Using Fluorine NMR Spectroscopy To Analyze Compounds
31P
29Si
11B
59Co
6,7Li
15N
What is Fluorine NMR?
Similar to proton (1H) NMR, fluorine-19 NMR is an analytical technique often used in chemistry labs. Unlike proton NMR which measures hydrogen atoms, fluorine NMR measures fluorine atoms. So researchers often rely on this technique to learn more about both organic compounds that contain fluorine (aka organofluorine compounds) and inorganic fluorine compounds.
What can you learn with 19F NMR?
Researchers use fluorine-19 NMR to figure out the molecular structure of fluorine containing compounds that they study. The properties and thus usefulness of the molecule they want to make will be closely related to its molecular structure. So they ask, “Did I succeed in incorporating fluorine in my compound? Is the fluorine atom in the right position in the molecule? Does this molecule have one, two, or more fluorine atoms?” 19F NMR helps answering questions like these.
A synthesis may take many steps to produce the target molecule or material. How do chemists know if each step resulted in the desired fluorinated compound? They can check by comparing the fluorine NMR spectra of the intermediate chemical product with the starting material. Fluorine NMR chemical shifts usually change as the molecular structure changes throughout a synthesis. Or, if the reaction incorporates a fluorine atom where one did not exist, then the 19F nmr spectrum will show a peak for that new fluorine atom.
If you are familiar with 1H NMR, you know that there are many different types of peaks in 1H NMR spectra. For example there are singlets, doublets, multiplets and so on (called NMR splitting patterns). This is because 1H nuclei interact (or couple) with each other. Coupling is one reason why NMR is useful in determining the connectivity in a molecule. Similarly 19F couples to itself as well as 1H and 13C, so you can determine where the fluorine atom is in the molecule by using so-called ‘coupling constant’.
Fun (not-fun) fluorine facts
Manhattan Project researchers used uranium hexafluoride as a key compound in the process to enrich uranium. The intense development of industries providing fluorine and fluorinated materials for atomic weapons facilitated an explosion of research in fluorine chemistry post-WW2.
Today, researchers exploit the unique characteristics of fluorine functional groups to control the chemical and physical properties of polymers, pharmaceuticals, agrochemicals, semiconductors and other beneficial chemical products. Fluorine’s ubiquity in advanced materials and its NMR-friendly nature have positioned it among the most studied nuclei in NMR spectroscopy.
Henri Moissan, a French chemist, won the 1906 Nobel Prize in Chemistry for isolating elemental fluorine.
19F Chemical Shift
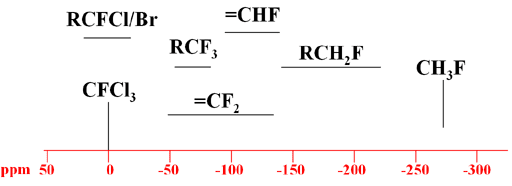
You probably know that the electron density around a proton determines its chemical shift. Where electron density is high, like in tetramethylsilane, the resonant frequency is low. Where the electron density is low, like in benzene, the resonant frequency is high. Fluorine 19 chemical shifts follow the opposite trend. Higher electron density leads to higher resonant frequency, and lower electron density, lower resonant frequency. Keep this point in mind when reading a fluorine nmr spectrum.
For example, consider three fluorine-containing functional groups, trifluoromethyl (R-CF3), difluoromethyl (R-CHF2) and fluoromethyl (R-CH2F). Due to fluorine’s electronegativity, the highest electron density among the three occurs in trifluoromethyl followed by difluoromethyl and then fluoromethyl. Consequently the fluorine nuclei in trifluoromethyl resonate at the highest frequency and those in fluoromethyl at the lowest frequency.
Why do these 1H and 19F nuclei behave differently as far as their chemical shifts are concerned? The s-orbital electrons around protons are diamagnetic. These diamagnetic electrons cause the proton nuclei to experience a reduced magnetic field(sometimes called the effective magnetic field or Beff). Consequently, they resonate at a lower frequency. However, some fluorine electrons (the p-orbital ones) are paramagnetic. These electrons cause fluorine nuclei to experience an increased magnetic field and therefore they resonate at a higher frequency.
Fortunately, fluorine NMR reference works, charts and tables can help you to read and understand your F19 spectra.
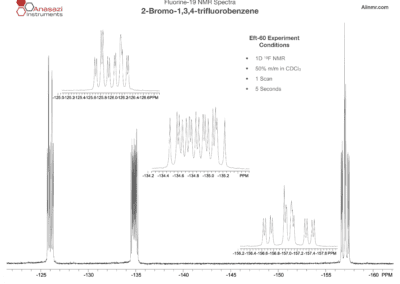
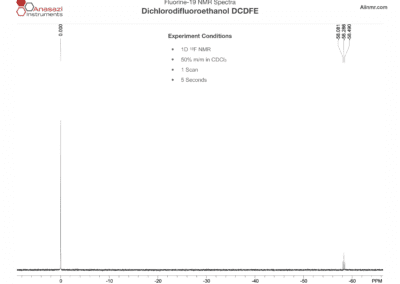
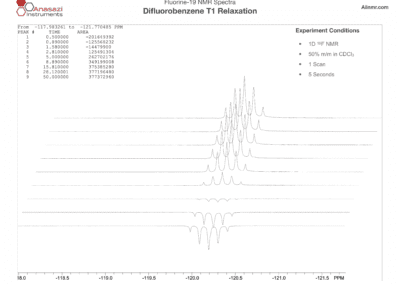
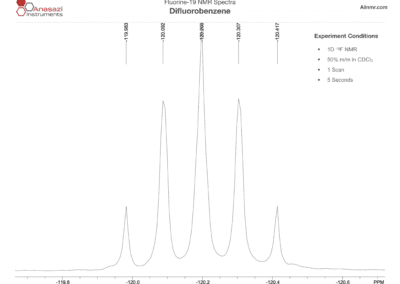
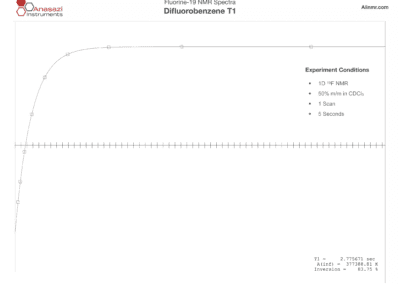
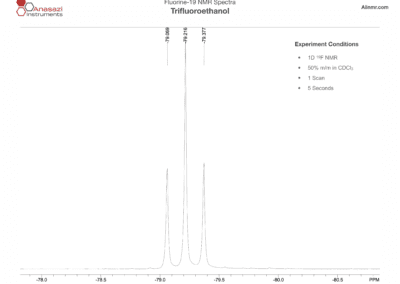
Fluorine-19 NMR Spectra
Recommended Literature
W. R. Dolbier, Guide to Fluorine NMR for Organic Chemists, John Wiley & Sons, Inc., New York, 2009
19F Properties
- 100% natural abundance
- Spin 1/2
- chemical shifts range of 800 ppm
- Receptivity 4730
- Gyromagnetic ratio 40.052 MHzT-1
Fluorine NMR standards
The most used fluorine reference standard is CFCl3. Dozens of others are also available.

Nuclei Series Download
To download a copy of Fluorine-19 for your use and reference click on the download PDF link below.