Using Lithium NMR Spectroscopy To Analyze Compounds
29Si
11B
59Co
15N
What can you do with 6,7Li NMR?
Study lithium ion solvation to optimize advanced inorganic materials for battery technologies. Measure J(6,7Li ,X) coupling constants to determine structure and connectivity of organolithium reagents.
Did You Know?
Some atomic weapons exploit the properties of the lithuim-6 nuclei. Because of the scale of industrial lithium-6 extraction, commercially available lithium is often enriched in lithium-7.
Lithium also has great utility in synthetic chemistry as part of organolithium reagents. For example, the carbon nuclei in a lithium carbon bond is nucleophilic and can add across a double bond creating a new carbon-carbon bond. The 1963 Nobel Prize winning chemist Karl Ziegler was a key contributor to organolithium chemistry.
Lithium is now one of the best know elements due to the ubiquity of lithium-ion batteries. John B. Goodenough, M. Stanley Whittingham, and Akira Yoshino won the 2019 Nobel Proze in chemistry for their contributions to the development of rechargeable lithium-ion batteries.
Recommended Literature
| H. Günther in Encyclopedia of Nuclear Magnetic Resonance, John Wiley & Sons, Inc., Chichester, 1996; Vol. 5, 2807-2825. | C. Detellier in NMR of Newly Accessible Nuclei, Academic Press, New York, 1983; Vol. 2, 105-151 |
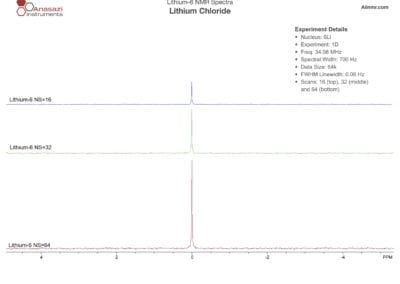
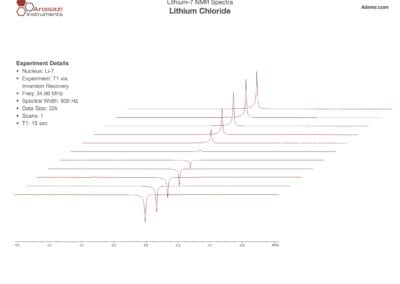
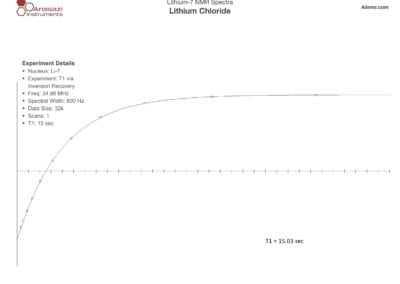
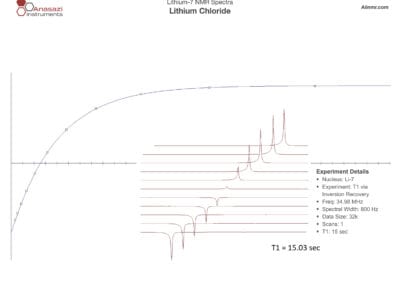
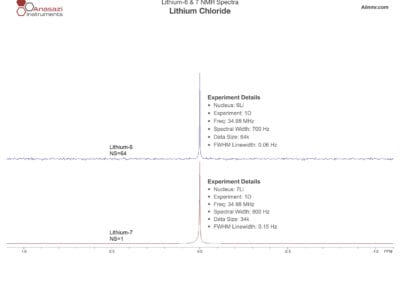
Lithium-6 & 7 NMR Spectra
Nuclei Series Download
To download a copy of Lithium-6 & 7 for your use and reference click on the download PDF link below.